Treatment for Rare Type of Soft Tissue Sarcoma
The FDA has approved a targeted therapy for epithelioid sarcoma, the first treatment approved for this form of cancer.
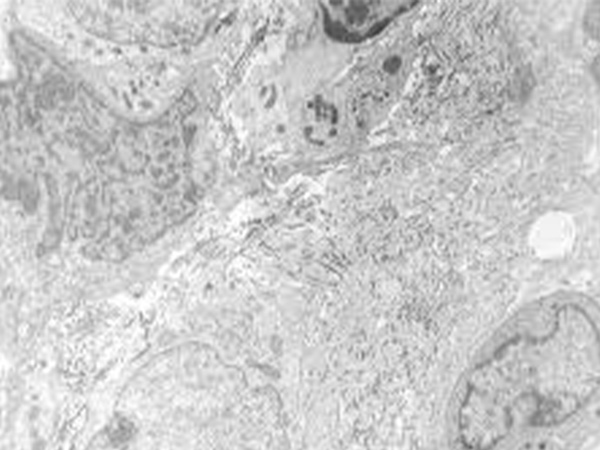
Epithelioid sarcoma is a rare form of soft tissue sarcoma, accounting for less than 1 percent of the approximately 12,700 people in the United States diagnosed with soft tissue sarcomas each year. On January 23, 2020, the U.S. Food and Drug Administration (FDA) approved tazemetostat (Tazverik) for treating patients age 16 and older with metastatic or locally advanced epithelioid sarcoma that cannot be completely removed by surgery.
The agency’s decision makes tazemetostat the first therapeutic approved specifically for treating this type of cancer.
Most cases of epithelioid sarcoma begin in the soft tissue under the skin of a finger, hand, forearm, lower leg, or foot. According to information in the FDA’s approval notice, about 50 percent of patients have metastatic disease at the time they are diagnosed with epithelioid sarcoma and this is considered a life-threatening diagnosis.
The approval of tazemetostat was based on results from a phase II clinical trial. The data showed that nine (15 percent) of the 62 patients with metastatic or locally advanced epithelioid sarcoma who received tazemetostat had partial or complete tumor shrinkage. The response lasted six months or longer for six of the nine patients who had a response.
Learn more about this approval on Cancer Research Catalyst, the official blog of the American Association for Cancer Research (AACR).