AACR Conference on the Microbiome, Viruses, and Cancer: How Gut Microbes Can Mediate Responses to Stem Cell Transplants
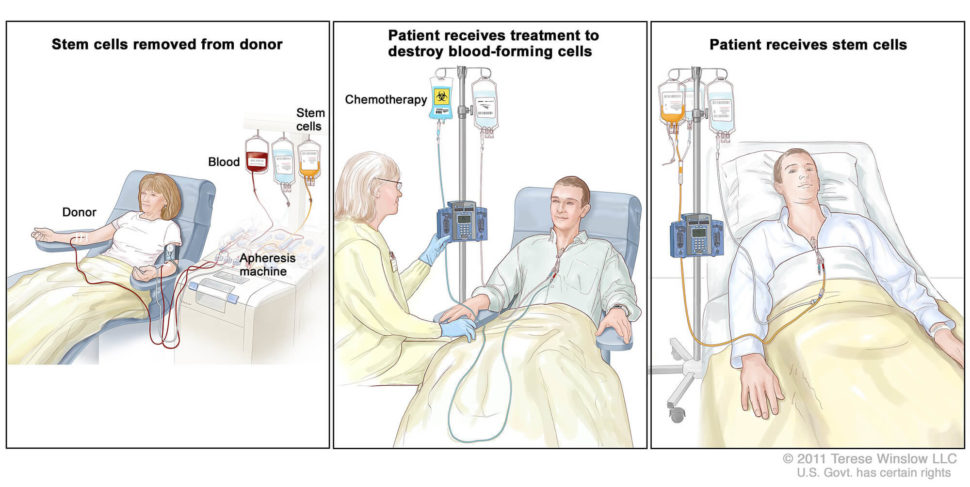
The first allogeneic hematopoietic-cell transplant (HCT)—a complex procedure where a patient receives blood stem cells isolated from a healthy donor—was performed in 1957. Now, over 9,000 allogeneic hematopoietic-cell transplants are performed per year in the United States. This type of transplant is generally used to treat patients with bone marrow disorders, including certain types of leukemia, lymphoma, and multiple myeloma.

“This is one of the few cancer therapies where we actually dare to say the words ‘trying to cure somebody,’ ” noted Marcel van den Brink, MD, PhD, during the second keynote address at the recent AACR special conference The Microbiome, Viruses, and Cancer.
A medical oncologist at Memorial Sloan Kettering Cancer Center in New York, van den Brink specializes in stem cell transplantation as a therapy for blood cancers. One major aspect of his research focuses on how the gut microbiome can modulate responses to this procedure, which he discussed in depth during his presentation at the conference.
Adverse outcomes following HCT are associated with specific gut microbial features
How is allogeneic HCT performed?
First, van den Brink explained, the recipient receives an intensive, cytotoxic conditioning regimen, consisting of total body irradiation, chemotherapy, or antibody therapies. The purpose of this regimen is two-fold: to debulk whatever tumor is still left in the patient, and to create space for the incoming stem cells. Following this conditioning treatment, the patient receives a stem cell graft from a matched donor.
There are four major causes of death following an allogeneic HCT. The most common cause of death is relapse of the blood cancer. Other adverse outcomes include infection, organ toxicity, and graft-versus-host disease (GvHD), a complication where the donor T cells view the host as foreign and attack the normal tissues of the patient. While multiple types of organs can be affected by GvHD, including the liver, skin, or brain, the most serious form of GvHD occurs in the gut, noted van den Brink.
Certain gut microbes are associated with outcomes following an allogeneic HCT – including overall survival. Increased abundance of bacteria from the genus Blautia, for example, has been shown to be associated with reduced GvHD mortality, and domination by gammaproteobacteria was found to be associated with increased pulmonary complications.
One factor that has been previously linked with increased overall survival following an allogeneic HCT is high gut microbial diversity. However, this finding came from a small, single-center study, van den Brink noted. To follow up on this research, van den Brink and colleagues conducted an international study, recently published in the New England Journal of Medicine, that interrogated whether gut microbiota could predict mortality in patients who received an allogeneic HCT.
Gut dysbiosis precedes transplantation in HCT recipients
In this study, the researchers analyzed nearly 9,000 stool samples from 1,361 HCT recipients from four transplantation centers. The bulk of these recipients were treated in New York (1,121 patients); the rest were treated in Durham, North Carolina (98 patients), Regensburg, Germany (76 patients), and Hokkaido, Japan (66 patients). Roughly half of the patients treated in New York received a T-cell-depleted allograft, which substantially decreases patients’ risk for developing GvHD.
The researchers analyzed baseline stool samples that had been obtained no more than 30 days before HCT. “The baseline flora was not that much different between the different centers and the different countries,” van den Brink noted, which he found surprising, as diets from these different areas are fairly divergent. One potential reason for this observation, hypothesized van den Brink, is the recipients’ pretreatment regimen, including antibiotics, which likely compromises the composition of their gut-flora.
To determine if HCT recipients had compromised microbial diversity before transplant, the researchers compared stool samples from roughly half of the study cohort taken at baseline with stool samples from nearly 250 healthy volunteers. They found that HCT recipients had significantly lower microbial diversity compared with the healthy population. Further, in the New York cohort, a lower baseline microbial diversity was associated with a higher risk of death following allogeneic HCT.
Microbial diversity declines following HCT and is predictive of overall survival
To assess changes in microbiota diversity following allogeneic HCT across the four different geographical regions, the researchers compared baseline stool samples with samples obtained in the periengraftment period (seven to 21 days following transplant) among evaluable patients. Across all four transplant centers, the researchers observed a loss in gut microbial diversity during this time frame.
Next, the researchers wanted to determine if there was an association between gut microbial diversity in the periengraftment period with overall survival. Compared with HCT recipients with lower gut diversity, HCT recipients with higher gut diversity in the periengraftment period had a lower risk of death. Among the patients treated in New York, only the patients who received unmodified (i.e. T-cell replete) grafts saw an association between microbial diversity and overall survival. This suggests that the correlation between gut microbial diversity and mortality is dependent on the presence of alloreactive T cells, van den Brink noted.
Based on these results, van den Brink proposed two distinct time points—before transplantation and during the periengraftment period—where strategies to modulate the gut microbiome could be evaluated in clinical trials of patients receiving allogeneic HCT.
Domination by Enterococcus can result in increased GvHD following HCT
The researchers also evaluated if the relative abundance of gut bacterial genera was altered after an allogeneic HCT. Indeed, they observed patterns of microbiota domination characterized by genus expansion following the procedure. The researchers quantified the occurrence of domination—defined as a relative-abundance threshold of any single taxonomic unit of at least 30 percent—during the transplantation period. Among all four cohorts, the cumulative incidence of domination rose similarly, typically peaking around one week after transplantation. One prominent genus that expanded dramatically was Enterococcus.
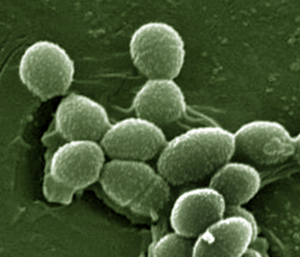
Enterococci are gram-positive, lactic acid bacteria that naturally occur in low abundance in the human gut. However, some species of Enterococcus are pathogenic and are associated with multidrug-resistant infections in patients. In a prior analysis of allogeneic HCT recipients at the same four transplantation centers, van den Brink and colleagues found that domination by Enterococcus in the early posttransplantation period (within 21 days after HCT) was associated with significantly reduced overall survival and increased GvHD-related mortality.
In the prior study, the researchers analyzed fecal samples from patients that had a highly diverse pretransplant gut microbiota and exhibited domination by Enterococcus following HCT using metagenomic sequencing. Compared with stool samples taken before transplant, posttransplant samples showed enrichment in pathways associated with lactose and galactose degradation.
The researchers found that cultured enterococcal strains associated with increased GvHD following HCT in either humans or mice did not grow when the media was depleted of lactose, and growth resumed once lactose was reinstated. This prompted the researchers to investigate whether modulating the diet of mice could affect outcomes following HCT. They found that mice fed with lactose-free chow had significantly reduced posttransplant expansion of Enterococcus in fecal samples and significantly increased overall survival.
To see if these findings translated to human patients, van den Brink and colleagues investigated whether HCT recipients with impaired lactose absorption had increased posttransplant expansion of Enterococcus. By analyzing germline DNA samples, the researchers could assess the patients’ lactose absorption and/or tolerance status based on a single-nucleotide polymorphism (SNP) that regulates lactase expression. Analysis of samples from approximately 600 patients showed that those with a lactose intolerance genotype had significantly prolonged enterococcal domination after cessation of antibiotics. These results suggest that diet modulation could attenuate the overgrowth of Enterococcus and potentially improve clinical outcomes in HCT recipients.
Food for thought
In addition to lactose modifications, van den Brink cited several other approaches that could modulate the gut microbiome for therapeutic benefit, including prebiotics (such as ingestion of non-digestible carbohydrates), postbiotics (such as avoidance of foods that compromise the mucus barrier), probiotics (including rationally selected bacterial strains), and antibiotics (such as commensal-sparing regimens).
While microbiome research remains an emerging area of study in oncology, it has become clear that these cohabitants in our gut play an important role in overall health. Understanding how the modulation of this diverse ecosystem—such as altering the composition or relative abundance of certain bacterial genera—can affect outcomes will likely continue to garner interest in the cancer research community.