Improving the Effectiveness of CAR T-cell Immunotherapy
A study presented in the AACR Annual Meeting 2018 media preview webinar, held March 15, examined closely a key ingredient necessary to make effective chimeric antigen receptor (CAR) T cells – the T cells of a patient. The researchers behind this study have identified one potential explanation for why we have not been successful in making CAR T-cell therapy work against solid tumors, and offer a solution.
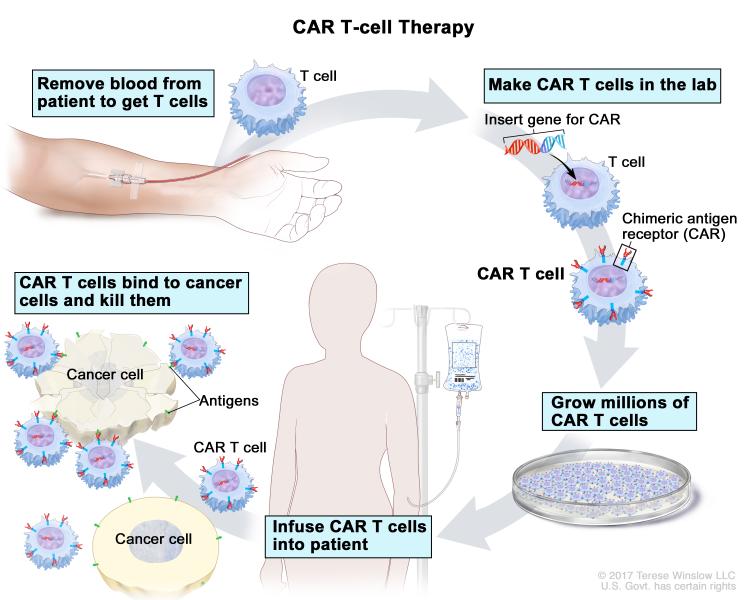
CAR T-cell therapy, a type of immunotherapy that is based on the principle of adoptive T-cell transfer, has revolutionized treatments for blood cancers, particularly in children. This therapy has yielded striking responses in some patients with cancers deemed incurable and in those that have stopped responding to chemotherapies, and resulted in complete remission rates of up to 90 percent in clinical trials.
Last year, the U.S. Food and Drug Administration approved two CAR T-cell therapies: tisagenlecleucel (Kymriah) was approved for treating certain pediatric and young adult patients with acute lymphoblastic leukemia (ALL), and axicabtagene ciloleucel (Yescarta) was approved for treating adults with certain types of non-Hodgkin lymphoma.
Unlike most cancer therapeutics that are “off the shelf” and administered intravenously or orally, treating patients with CAR T-cell therapy is a much more involved process. There are multiple, highly specialized steps, including isolating T cells from a patient’s body, genetically modifying them to express a CAR that is targeted toward a protein present in the cancer cells, and reintroducing them in to the patient’s body so the “equipped” T cells multiply and attack the cancer cells.
Like most cancer treatments, this type of therapy is not without setbacks. Despite the high success rates in treating certain blood cancers with CAR T-cell therapy, there is a small population of patients that do not benefit from this therapy, and some patients develop resistance to this treatment. Further, efforts to make this therapy work for solid tumors have not been very successful so far for a variety of reasons, as described in this review.
Several research teams are attempting to tinker with CAR T-cells to improve their efficacy but also to make them work against solid tumors. Efforts include fine-tuning CAR T cells, remote-controlling them with an “on” and “off” switch, and using a universal adaptor. In a study published in Nature Communications this month, researchers describe a non-invasive imaging tool to repeatedly image CAR T cells in vivo and determine their location and distribution in the body over time that could enable a better understanding of the relationship between CAR T-cell accumulation and disease control.
Examining the starting material
One approach to improving CAR T-cell therapy and potentially making it effective against solid tumors involves going back to the drawing board and looking at the starting material, according to research presented in the AACR Annual Meeting 2018 media preview webinar by David M. Barrett, MD, PhD, assistant professor of pediatrics at Children’s Hospital of Philadelphia.
“We were interested in learning what made good starting material for CAR T manufacture, rather than repeating prior work on learning what a good CAR T cell looks like after it has been made,” said Barrett, in an interview.
In this study, co-sponsored by an Stand Up To Cancer (SU2C) Innovative Research Grant (the AACR is the Scientific Partner of SU2C) Barrett and colleagues found that a lot can be learned by closely examining the patient T cells, which are an essential starting material for the process of CAR T-cell manufacture.
An important criterion for modifying T cells into CAR T cells is that the T cells from the patient must be healthy enough to survive during the complex endeavor of making them express a CAR, and then have enough energy left once reintroduced in to the patient to efficiently attack the cancer cells.
“In several of the patients with leukemia we first attempted to treat, we noticed the T cells looked exhausted when we first collected them and they either did not survive the lab process to turn them into CAR T cells or did not have enough energy left to work in the patient as a result,” Barrett said.
Barrett and team set out to understand why some children’s T cells were of poor quality.
The metabolism of normal T cells changes when they are stimulated to convert into a CAR T cell, Barrett explained. Normal T cells rely heavily on the fuel sources in the environment. They use one of the two fuel sources, the glycolysis or the glutamine and fatty acid pathway, to build their energy. The researchers learned that while T cells that use glutamine and fatty acid pathways as fuel sources had great CAR T-cell potential, those that depended on glycolysis were poorly suited to the process of CAR T-cell manufacture.
Chemotherapy may impair T cells’ potential to become effective CAR T cells
The researchers examined the T cells in 157 pediatric patients with blood cancers (ALL, chronic myelogenous leukemia, non-Hodgkin lymphoma, or Hodgkin lymphoma) or solid tumors (neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms tumor, or Ewing sarcoma) at diagnosis and after each cycle of chemotherapy.
The CAR T-cell potential of the T cells was very poor in all tumor types except ALL and Wilms tumor in the pre-chemotherapy samples, and there was a decline in CAR T-cell potential with cumulative chemotherapy in all cancer types. Nanostring RNA profiling of metabolic pathways revealed T cells with poor CAR T-cell potential were biased toward glycolysis as their fuel source instead of fatty acids.
Analysis of oxygen consumption rate also showed that certain classes of chemotherapies, particularly those regimens that had cyclophosphamide and doxorubicin, were harmful to the “spare respiratory capacity” (SRC) of T cells. “SRC is a measure of the energy reserve of a cell, and is based on how many mitochondria are present and how healthy they are,” Barrett explained. Having a poor energy reserve means the T cell is likely to either be inefficient to begin with or to die before it could kill the cancer cells.
“Based on these data, we have altered our practice for T-cell collection for children with leukemia, for children with high-risk disease … we will collect T cells early even if that patient is not currently eligible for CAR trial simply because we know that cumulative therapy is going to progressively deteriorate the likelihood that the cells will make a functional CAR product,” Barrett noted in the webinar. “We have been recommending that to other centers.”
Fixing the CAR T-cell manufacturing process
Further studies by Barrett and colleagues showed that it is possible to force-feed the T cells with fatty acids, the preferred source of fuel, to restore the SRC in chemotherapy-exposed T cells.
“We have gotten CAR T cells to work for leukemias but not yet been very successful in solid tumors. There are a number of potential reasons for this, but our data suggest poor T-cell starting material may be a key first problem,” Barrett noted. “The T cells from solid tumor patients may need different manufacturing protocols to be successful.”