Illuminating metabolism: A pioneering approach to combat pancreatic cancer
By Alice Bradbury, PhD
Healthy cells rely on various chemical reactions that manage the conversion of food into energy and the materials needed to grow and reproduce. As a biochemist, Costas Lyssiotis, PhD, was always fascinated by these processes, known collectively as metabolism.

In cancer cells, these metabolic processes are hijacked and reprogrammed to allow the cancer to grow and thrive.
After obtaining his doctorate from the Scripps Research Institute, Dr. Lyssiotis joined the lab of Lewis C. Cantley, PhD, at Harvard Medical School and Weill Cornell to study these cancer-specific metabolic processes.
It was during this time as a postdoctoral researcher that he applied for and was awarded the 2013 Pancreatic Cancer Action Network-AACR Pathway to Leadership Grant.
His project aimed to investigate the mechanisms by which metabolites support the growth of pancreatic cancer, a disease that is unusually resistant to therapies.
In pancreatic cancer, “The tumors are hyperdense and hypovascular, which impacts how the tumor cells find food. We think these metabolic features are part and parcel of their therapeutic resistance,” Dr. Lyssiotis explained.
A unique funding opportunity, the Pathway to Leadership grant was designed to support the transition of mentored pancreatic cancer researchers to independent faculty by providing funding for two years of mentored research, followed by three years of independent research.
“Receipt of this prestigious award provided protected support to build out my ideas in this area, which facilitated my attainment of a faculty position,” he said. “This grant was one of the most important contributors to my career interests and career trajectory. I cannot sufficiently express in words how grateful and honored I was to receive it.”
Dr. Lyssiotis began his assistant professorship at the University of Michigan in 2015 and was awarded another two AACR grants shortly after.

In 2016, he was awarded the AACR-Bayer Innovation and Discovery award before receiving the AACR NextGen Grant for Transformative Cancer Research in 2017.
“The NextGen award provided the burgeoning Lyssiotis laboratory with support to pursue high-risk, high-reward ideas, and ultimately to establish several of the now founding ideas for our group,” he said.
The support from the grant also helped provide stability for the Lyssiotis laboratory to grow.
“It has been instrumental in our longer-term success,” he said, adding “The initial studies/data generated with support from the NextGen grant were leveraged to win numerous extramural awards, including an R37 and two R01s, resulted in invited seminars at symposia, and from this publicity, recruitment of young, bright scientists into the study of pancreatic cancer metabolism.”
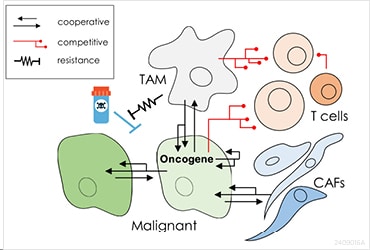
With funding from the AACR, Dr. Lyssiotis and his team have discovered several mechanisms by which metabolites support tumor growth, immune evasion, and resistance to therapy.
Explaining the significance of their discoveries he said, “The illumination of these concepts and pathways have revealed new druggable targets and pathways, and we are excited to support their continued development for pancreatic cancer treatments.”
Now a full Professor at the University of Michigan and looking to the future, Dr. Lyssiotis believes that understanding and controlling the immune system holds the promise to cure pancreatic cancer.
“Metabolism plays an absolutely central role in these processes, as antitumor immune cells (like cancer cells) require nutrients to make more immune cells and to carry out tumor killing functions. Cancer cells know this and actively deplete nutrients to reduce the activity of the anti-tumor immune system,” he said. “Thus, metabolism-based approaches (which may include diet) will be critical to bring the full power of the immune system to bear against pancreatic cancer.”